How a potent psychoactive drug synthesized?
The psychoactive substance psilocybin is the most important natural product of so-called “magic mushrooms” of the genus Psilocybe, which makes these mushrooms a popular drug. However, psilocybin has also become increasingly interesting in medicine in recent years for a number of mental illnesses. It has shown promising results in the treatment of depression, addiction and anxiety. Psilocybin is therefore already at an advanced stage of clinical testing as an active pharmaceutical ingredient.
Psilocybin is formed by fungi in complex biochemical processes from the amino acid L-tryptophan. The enzyme PsiM, a methyltransferase, plays an important role in this process. It catalyzes two methylation reactions in succession, the last two steps in the production of psilocybin: “There are many methyl transfer reactions in nature,” says the senior author. “Here, we asked ourselves how exactly psilocybin production is accomplished.”
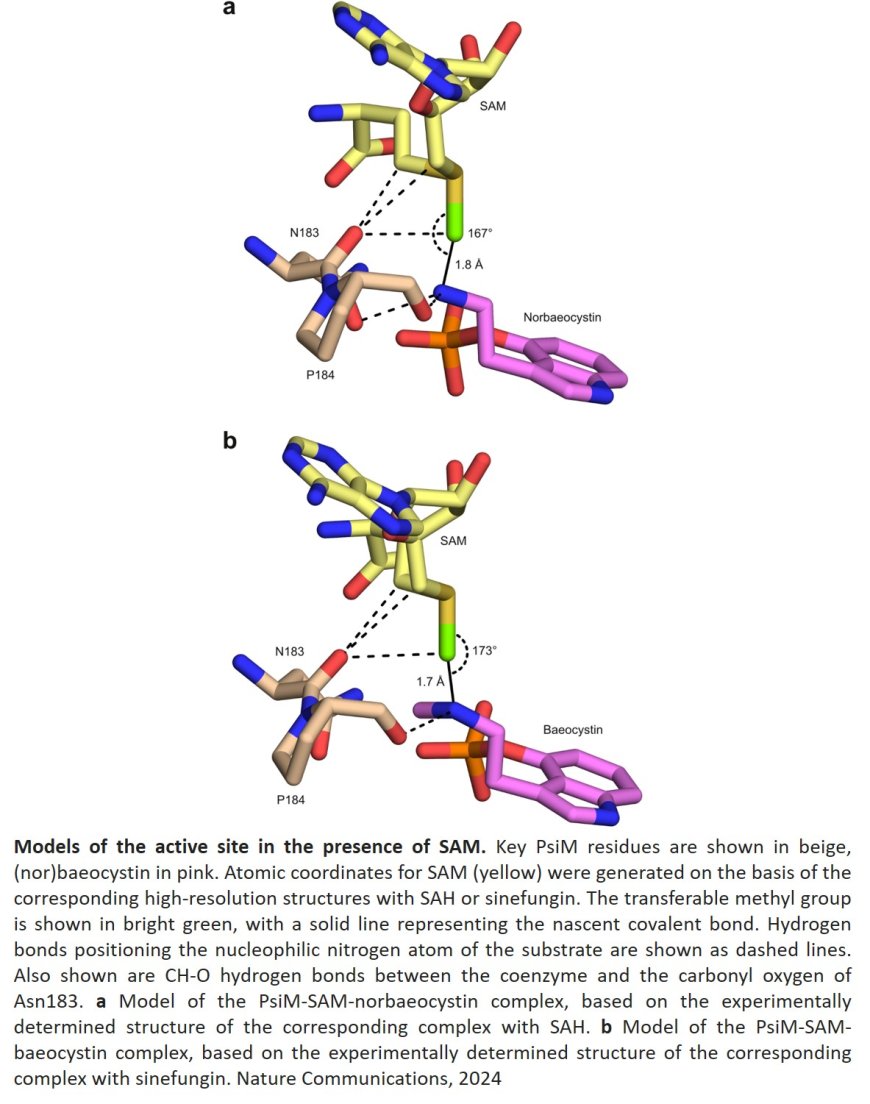
To this end, a team of researchers investigated the enzyme PsiM both biochemically and using X-ray crystal structure analysis. This method allows proteins to be visualized down to the atomic level, whereby several stages of the reaction could be depicted in ultra-high resolution.
Examination of the protein structure revealed astonishing similarities in structure between the fungal enzyme PsiM and enzymes that are normally responsible for the modification of RNA. Although there are also differences, the great structural similarity indicates that the fungal enzyme has evolved from a single methylating RNA methyltransferase. Accordingly, it previously only had the ability to attach a single methyl group to the target molecule. “The psilocybin precursor norbaeocystin, which is converted by PsiM, structurally imitates part of the RNA, but is methylated twice,” says the author.
In further investigations, the researchers were also able to identify a crucial amino acid exchange that gave PsiM the ability to carry out double methylation during evolution. This process involves the final step in the entire reaction chain for potential biotechnological production of the active ingredient: the conversion of the single-methylated intermediate baeocystin to the double-methylated psilocybin.
The researchers then wondered whether PsiM could also convert psilocybin to aeruginascin by attaching a third methyl group. Aeruginascin is an analog of psilocybin, which occurs naturally in some types of fungi. “The only question is, where does it come from?” asks the author.
Until now, there has been disagreement in the scientific community as to whether the compound is a metabolic product of the psilocybin biosynthesis pathway and could arise from psilocybin through PsiM. The study now provides a clear result: “This is clearly not the case,” says the author. “PsiM is not able to convert psilocybin to aeruginascin.” PsiM can therefore be ruled out for the biosynthetic production of this analog. However, the enzyme could be relevant for the production of psilocybin in microorganisms in the future: “Overall, our results can help to develop new variants of psilocybin with improved therapeutic properties and to produce them biotechnologically,” says the author.