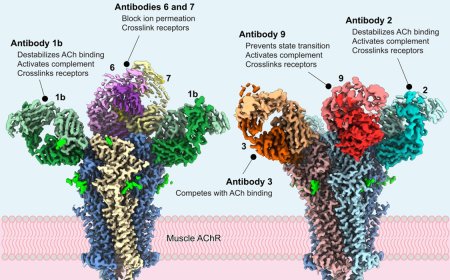
Interplay of various enzymes in innate immunity
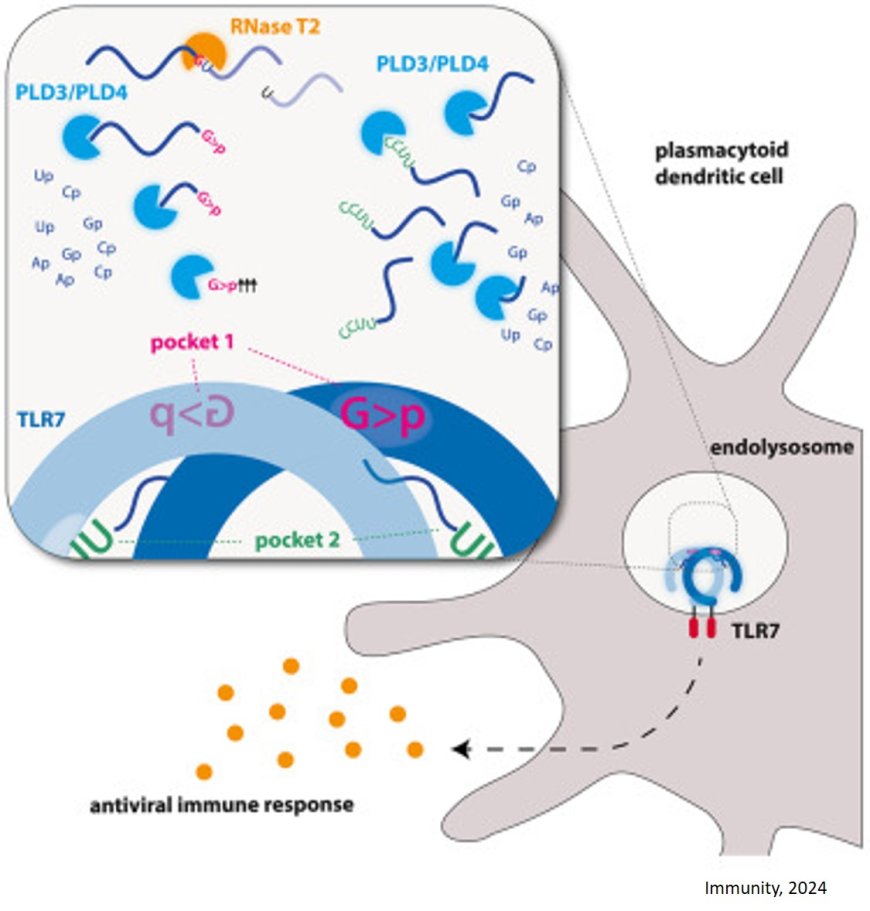
Toll-like receptor 7 (TLR7), located in the dendritic cells of our immune system, plays a crucial role in our natural defense against viruses. TLR7 recognizes single-stranded viral and other foreign RNA and activates the release of inflammatory mediators. Dysfunctions of this receptor also play a key role in autoimmune diseases, making it all the more important to understand, and ideally modulate, the exact activation mechanism of TLR7.
Researchers have now managed to gain deeper insights into the complex activation mechanism. It was known from earlier studies that complex RNA molecules have to be cut up first so that the receptor is able to recognize them. Using a wide range of technologies from cell biology to cryogenic electron microscopy, the researchers have revealed how single-stranded foreign RNA is processed to be detected by TLR7. Their work has been published in the journal Immunity.
In the course of evolution, the immune system has specialized in recognizing pathogens from their genetic material. For example, the innate immune receptor TLR7 is stimulated by viral RNA. We can picture viral RNAs as long threads of molecules, which are much too large to be recognized as ligands for TLR7. This is where nucleases come in – molecular cutting tools that chop the ‘RNA thread’ into small pieces. Endonucleases cut the RNA molecules through the middle like scissors, while exonucleases cleave the thread from one end to the other. This process generates various RNA snippets, which can now bind to two different pockets of the TLR7 receptor. Only once both binding pockets of the receptor are occupied by these RNA pieces a signaling cascade set in motion, which activates the cell and triggers a state of alarm.
The researchers discovered that RNA recognition by TLR7 requires the activity of the endonuclease RNase T2 operating in conjunction with the exonucleases PLD3 and PLD4 (phospholipase D3 and D4). “Although it was known that these enzymes can degrade RNAs,” says the author, “we have now demonstrated that they interact and thereby activate TLR7.”
The researchers also found that the PLD exonucleases have a dual role within immune cells. In the case of TLR7, they have a pro-inflammatory effect, whereas in the case of another TLR receptor, TLR9, they have an anti-inflammatory effect. “This dual role of PLD exonucleases points to a finely coordinated balance for controlling appropriate immune responses,” explains the author.
“The simultaneous promotion and inhibition of inflammation by these enzymes could serve as an important protective mechanism for preventing dysfunctions arising in the system.” What role other enzymes could have on this signaling pathway and whether the molecules involved are suitable as target structures for therapies are subjects for further investigations.