Nanoparticle delivery of FZD4 to lung endothelial cells inhibits lung cancer progression and metastases
A recent breakthrough study from the lab has shown potential to improve therapeutic outcomes for patients suffering from lung cancers.
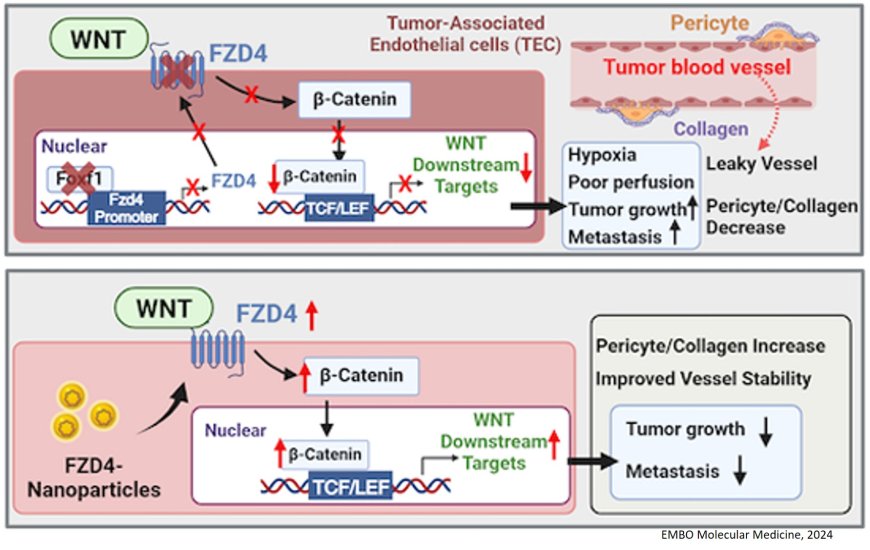
“We have identified the novel protein FOXF1 that stabilizes blood vessels inside the lung tumors, decreases intertumoral hypoxia and prevents lung cancer metastases,” explained the senior author on this study.
Lung cancer remains the leading cause of cancer-related mortality worldwide, according to the American Lung Association. In 2021 alone, the disease accounted for 22% of all cancer deaths. With less than a 20% five-year survival rate for patients with advanced non-small cell lung cancers, a promising treatment approach like this is desperately needed.
In pursuit of more therapeutic approaches, the lab developed a nanoparticle delivery system to successfully deliver FZD4 to pulmonary endothelium, which decreased lung tumor growth and metastasis in pre-clinical models of lung cancer. Thus, increasing levels of FOXF1 or FZD4 — either genetically or via gene therapy — shows promise to improve therapeutic outcomes in lung cancer patients.
The studies from the group support the use of FOXF1 — or FZD4-activating — therapies to enhance the delivery of chemotherapeutic agents or immune checkpoint inhibitors during lung cancer treatment.
“Since targeting the FOXF1/FZD4 signaling using gene therapy had efficiently decreased lung cancer progression and normalized tumor blood vessels, our next step will be to develop pharmacological approach to activate this signaling pathway and to move this therapy into clinical trials,” the author said.
The manuscript demonstrated that FOXF1 is expressed in normal lung endothelial cells, but it is decreased in the tumor-associated vasculature of lung cancers. Using the Cancer Genome Atlas datasets, they showed that lung cancer patients with higher FOXF1 mRNA expression had increased survival compared to those with lower FOXF1 levels.
The team then actively removed the FOXF1 gene from endothelial cells, using gene-editing technology. The effects of this were staggering. Removal of FOXF1 in their models promoted lung tumor growth and metastasis; caused functional and structural abnormalities in tumor vasculature; and led to a lack of frizzled-4 (FZD4) — a gene that participates in the Wnt/β-catenin signaling pathway, enacting a series of steps that affect the way cells and tissues develop.
Next, they increased FOXF1 gene expression in endothelial cells using a transgenic model of lung cancer. By increasing FOXF1 levels, they effectively inhibited lung tumor growth and metastasis, and stabilized tumor-associated blood vessels. They have also shown that FOXF1 directly activated FZD4, one of the Wnt/β-catenin signaling receptors.