Science Hangouts
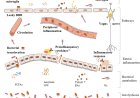
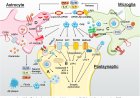
Protein monoaminylation in brain: novel mechanisms of neural development, plasticity and disease
9288

Science Hangouts
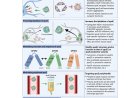
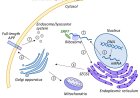
Digestive Enzymes On the Loose: Autodigestion in Metabolic Disease, Shock and Death
237

Science Hangouts
Protein monoaminylation in brain: novel mechanisms of neural development, plasticity and disease
9288

Science Hangouts
Digestive Enzymes On the Loose: Autodigestion in Metabolic Disease, Shock and Death
237